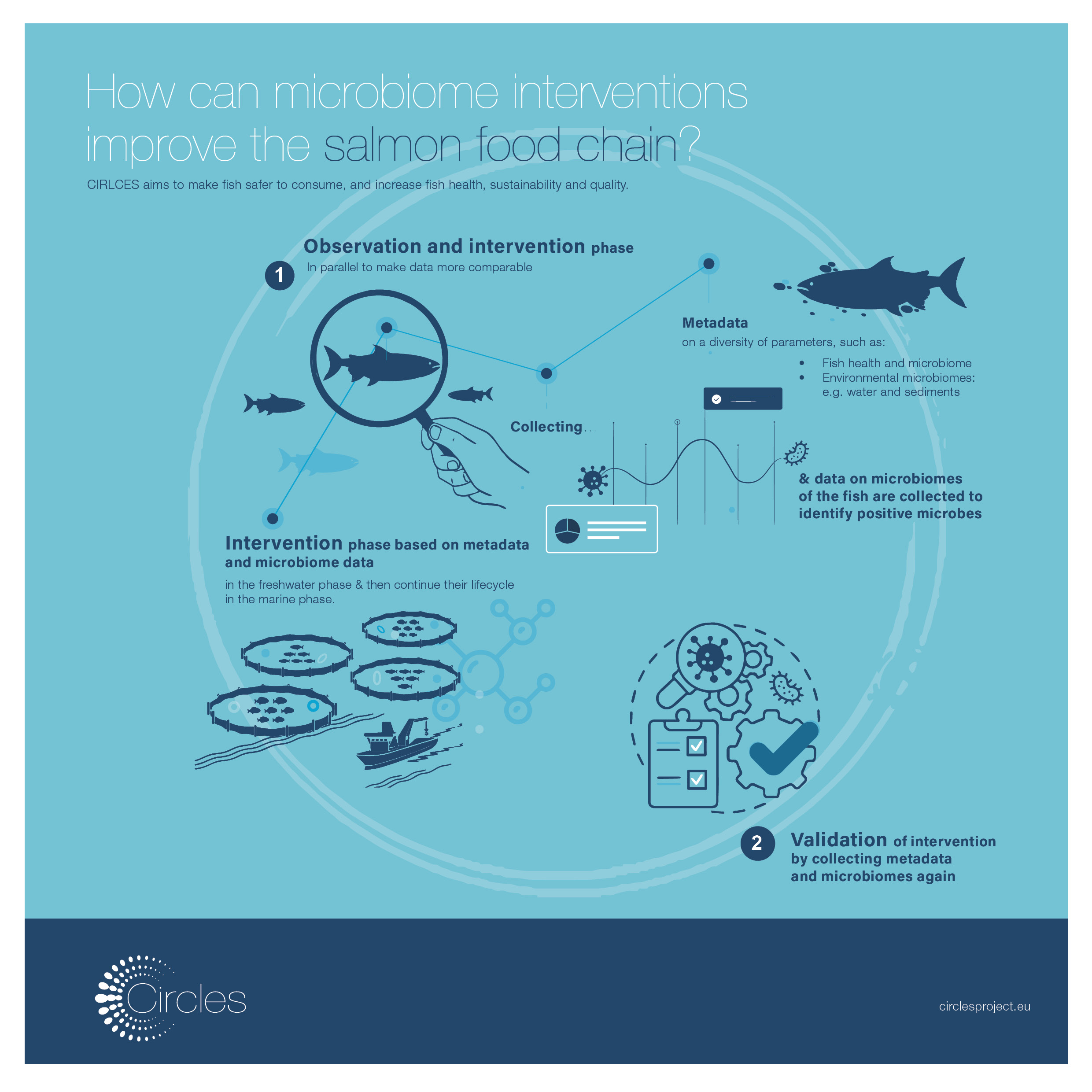
After several years of relentless work, a team of researchers – part of which is involved in the CIRCLES project – have been successful in the characterisation of the Neanderthal gut microbiome. Scientists sequenced the DNA from 50.000-year-old human faeces. The discovery has important implications on human health and evolutionary medicine.
Because of a continuous and rapid increase of chronic inflammatory diseases in the West, there has been a great interest in understanding the components of the gut microbiota we cannot afford to lose without compromising our health. By comparing the structure and composition of the gut microbiota across populations with varying subsistence and lifestyle features (semi-nomadic hunting and gathering, rural farming, nomadic pastoralism, urban industrial agriculture), researchers found a prevailing reduction of bacterial diversity in the gut microbiota among groups in the category ‘urban industrial agriculture’.
The loss of bacterial diversity concerns a series of microorganisms referred to as “old friends”. The accelerated change of the gut microbiota can probably be attributed to factors typical of hyper-industrialised urbanisation, such as the frequent use of pharmaceuticals, highly sanitised environments, the consumption of intensively cultivated and processed foods, and the exposure to xenobiotics. Although the loss of “old friends” may not be a problem for our health, the process of depletion of the gut microbiota in westernised (urban) populations may represent an indicator for the course of the disease, especially if it involves the loss of microbial components crucial for our physiology.
Through the analysis of ancient DNA at the archaeological site of El Salt in Alicante, Spain, researchers have now been able to reconstruct the profile of the Neanderthal gut microbiota, identifying a core of microorganisms shared with modern humans. Among these microorganisms, they have identified well-known fibrolytic bacteria capable of producing short-chain fatty acids, thus demonstrating their potential as keystones to the health of our species. Furthermore, in the Neanderthal gut microbiota, researchers have identified the so-called “old friends”, thus confirming the hypothesis about their ancestral nature as components of the human gut microbiota that become lost through various factors of post-industrialized lifestyles.
Correspondence between features found in the El Salt samples to those of modern humans would support the view that humans and Neanderthals are both dependent on similar gut microbial arrangements, possibly due to their developmental precedence in the gut of an earlier common ancestor, perhaps more than one million years ago. The information gathered in this research could guide therapeutic or disease mitigation strategies, based on diet and lifestyle, including the revision of the use of xenobiotics, to promote retaining the members of the gut microbiota that are fundamental to our health.
Full article available here.